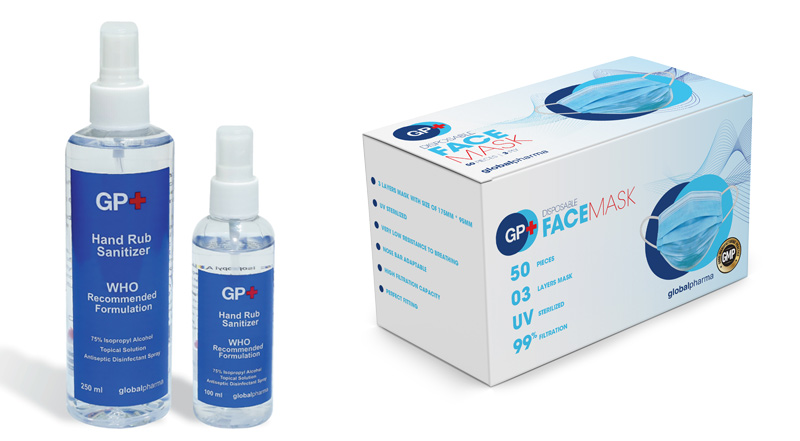
Globalpharma, a wholly-owned subsidiary of Dubai Investments PJSC, has announced the launch of its first UAE made hand rub sanitizer and surgical disposable face masks under the brand “GP+”. The GP+ hand sanitizers will be available across pharmacies in the UAE by the end of this month and the mass manufactured surgical disposable face masks in the coming weeks.
This comes after the latest Ministry of Health and Prevention (MOHAP) guidance & support to local manufacturers, granting accelerated approval and facilitating availability of raw materials with highest standards.
The GP+ sanitizer is approved by the Dubai Municipality and manufactured in accordance with the latest guidelines as outlined by the World Health Organization (WHO). The formulation offers a quick and persistent action and helps protect against many enveloped and non-enveloped viruses and microorganisms. The facemasks are made with high quality melt blown filter offering highest filtration rate; and produced under UV sterilization at the Globalpharma premises that is applying the GMP.
“There has been a significant acceleration in the demand for hand sanitizers owing to the prevalence of infections propelling the need for sanitizers. As a responsible pharmaceutical company, we are focused towards supporting market demands and the launch of GP+ is aimed at addressing the need for trustworthy and preventive hygiene solutions to meet the needs of the nation in combatting the spread of the infections”, said Mohammed Saeed Al Raqbani, General Manager, Dubai Investments Industries.
With productions at Globalpharma’ s local factories in the UAE, the Company continues to evaluate and study the possibilities of manufacturing and supplying medical supplies, aimed at supporting demanding medical emergencies.
“The manufacturing of the sanitizers and the surgical disposable face masks have followed the highest quality of good manufacturing practice (GMP) standards, ensuring highest quality products. We developed formulas in accordance with the regulations and manufacturing is progressing steadily at our local facilities, scaled up to produce larger quantities. The packaging and testing of the products has been ongoing over the last few weeks. The sanitizer solution consists of 75% Isopropyl alcohol and is available in 100ml and 250ml spray bottles, having the capacity to promote improved compliance with hand hygiene by making the process faster and more convenient”, said Basem Al Barahmeh, General Manager, Globalpharma Co. L.L.C.
As a responsible pharmaceutical company, Globalpharma is currently leveraging distribution networks as well as modern trade channels to make the product easily available to meet the rising demand for sanitizers across the country.
Globalpharma manufactures and commercializes pharmaceutical products under CGMP (current good manufacturing practice) conditions including antibiotics, cardiovascular, anti-ulcerants, analgesic, NSAIDs (Nonsteroidal anti-inflammatory medicines), food supplements, vitamins, anti-diabetics, respiratory products and anti-histamine formulations.